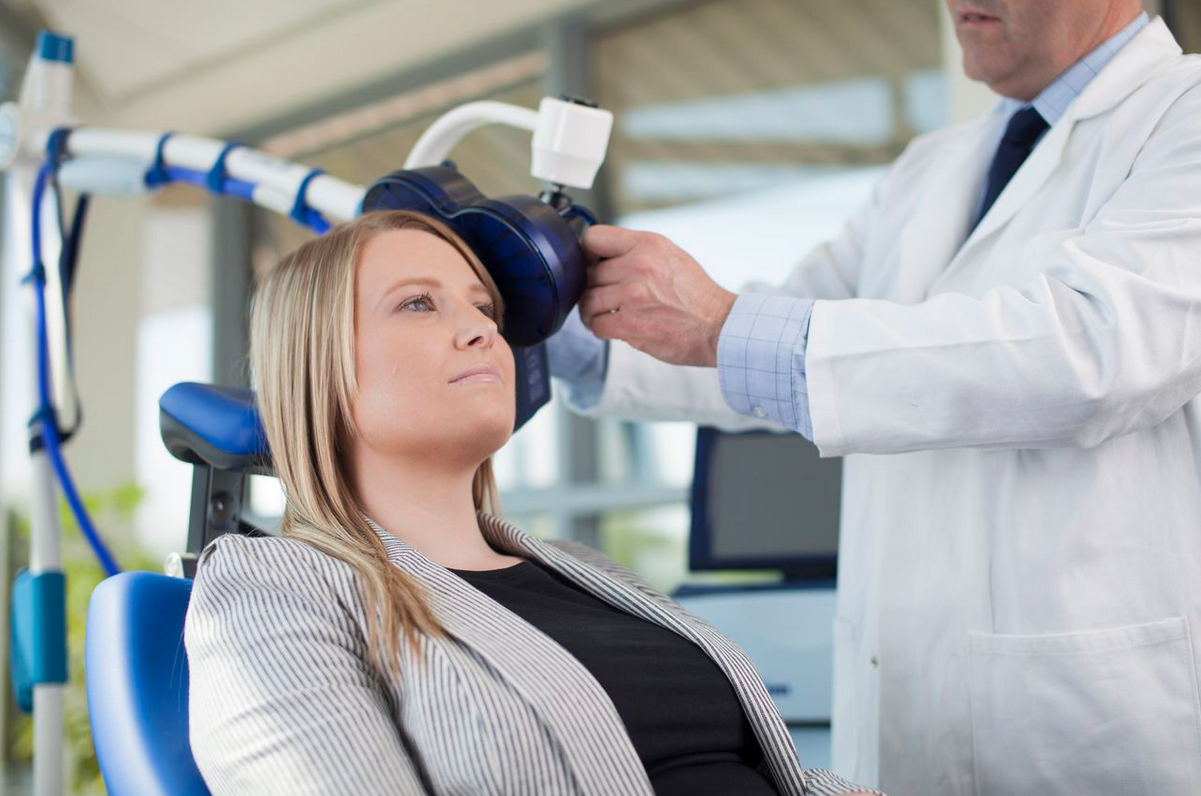
Repetitive Transcranial Magnetic Stimulation
A non-invasive neuromodulation technique
- CENALIFE
- CENALIFE
- CENALIFE
- CENALIFE
- CENALIFE
- CENALIFE
- CENALIFE
- CENALIFE
- CENALIFE
Our Approach

Advantages
rTMS produces very few adverse effects and is better tolerated than medications or other therapeutic neuromodulation approaches. It specifically increases connections between areas of the brain that are seen to have gaps in patients with mental health illness and treatment-resistant disease.
If you’re a new client, please take some time to review these considerations before booking an Info Session.
- Program
- Pricing
- Program
- Pricing
- Program
- Pricing
- Program
- Pricing
- Program
rTMS Program & Pricing
If you’re a new client, please take some time to review these considerations before booking an Info Session.
- FAQ
- FAQ
- FAQ
- FAQ
- FAQ
- FAQ
- FAQ
- FAQ
- FAQ
- FAQ
- FAQ
- FAQ
- FAQ
rTMS FAQs
Transcranial Magnetic Stimulation (TMS) is a non-invasive procedure that uses magnetic fields to stimulate nerve cells in the brain to improve symptoms of depression and other conditions, such as anxiety, PTSD, OCD and pain.
rTMS is designed for individuals who cannot tolerate or have limited response to medications and other mental health treatments.
There is recent evidence that rTMS helps with pain disorders, and mild cognitive impairment (pre-dementia). It can also be used when other treatment (such as medications) have failed.
In patients with depression who have failed multiple antidepressants, approximately 30-40% can be completely free of depressive symptoms with rTMS, and up to 70% will have some improvement in symptoms. rTMS may even be more effective in patients who are not treatment-resistant. Recent systematic reviews confirm rTMS to be significantly beneficial in anxiety disorders and PTSD.
Minimal. Most people tolerate rTMS without any significant side effects. Common side effects may include: Headache Scalp discomfort at the site of stimulation Tingling, spasms or twitching of facial muscles Lightheadedness Very rare side effects can include Risk of seizure during treatment (1 in 1000 theoretical risk) Risk of mania in patients with bipolar disorderSide effects are generally mild to moderate and improve shortly after an individual session and decrease over time with additional sessions.
If you have any type of ferromagnetic metal in your head, you are not eligible for TMS therapy. This includes aneurysm clips or coils, stents in the neck or brain, implanted electrodes, deep brain stimulators, metal plates, or metallic implants. Braces, dental fillings, or titanium are OK. To discuss other eligibility requirements including history of seizures or substance use, please contact us.
There are no medications that interact with TMS therapy. Let your provider know if your medication regimen changes at any time during treatment.